Safety and Efficacy of
Chlorine Dioxide Gas
The specific oxidation effect of chlorine dioxide
(molecular formula: ClO2) changes the structure of
the targets (viruses and bacteria) and reduces their functioning.
As a result, it demonstrates three effects.
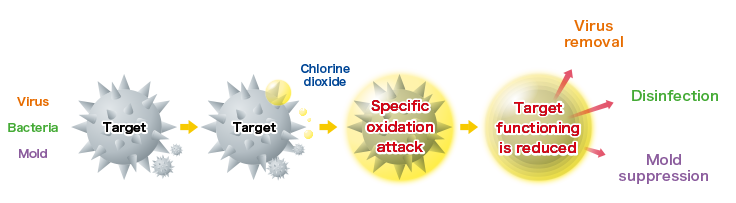
(Image is for illustration purposes.)
Note: This page provides research information on chlorine dioxide. It is not product information.
Note: This page provides research information on chlorine dioxide. It is not product information.
What is chlorine dioxide?
Chlorine dioxide exists as orange- to yellow-colored heavier-than-air gas at high concentrations. It produces irritating odor similar to that of chlorine. Since chlorine dioxide is a type of radical that has a strong oxidizing power, it is known to have functions, including virus-eliminating action, bacteria-eradicating action and antimycotic action.
Chlorine dioxide is soluble in water and is used for these purposes, such as bacterial eradication, as a form of chlorine dioxide solution.
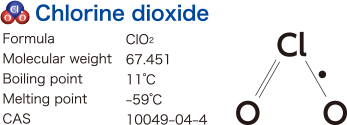
Chlorine dioxide used in various fields
Chlorine dioxide is approved for disinfection of water and as a food additive for bleaching wheat flour in Japan and the United States 1-4). In China, chlorine dioxide was approved as a disinfectant against COVID-19 for the following purposes (according to a notification in February 2020): disinfection of water (for drinking water and for hospital sewage water), object surfaces, kitchen instruments, food and processing tools and equipment, fruits, vegetables, medical instruments (including endoscopes), and air 5). However, chlorine dioxide is not approved as a pharmaceutical product or a quasi-drug disinfectant in Japan.
- 1) U.S. Food and Drug Administration (FDA), 21CFR§173.300, 21CFR§137.105, 21CFR§137.200, 21CFR§165.110, 7CFR§205.601, 7CFR§205.603, 7CFR§205.605
- 2) U.S. Environment Protection Agency (EPA), National Primary Drinking Water Regulations
- 3) Ministerial ordinance for technical standard of Water Supply
- 4) Notification No. 370, Standards for foods, additives by Ministry of Health of Japan
- 5) Notice issued by Office of National Health Commission on printing and distributing the guidelines for using disinfectants. Supervision Letter of the National Health Commission Office [2020] No. 147, Guideline for using Disinfectants February 2020.
Elucidation of infection prevention mechanisms of chlorine dioxide
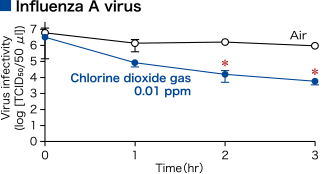
Influenza virus
Specific oxidation of amino acid tyrosine and tryptophan constituting the spike protein hemagglutinin (HA) on the virus surfaceChlorine dioxide was demonstrated to specifically act on and oxidize the amino acid tyrosine and tryptophan constituting the hemagglutinin (HA) on the surface of an influenza virus (H1N1). Furthermore, it was confirmed that chlorine dioxide oxidizes the amino acid tryptophan 153 residue, which constitutes the receptor-binding site on hemagglutinin (HA) of infected cells, converting it to N-formylkynurenine.
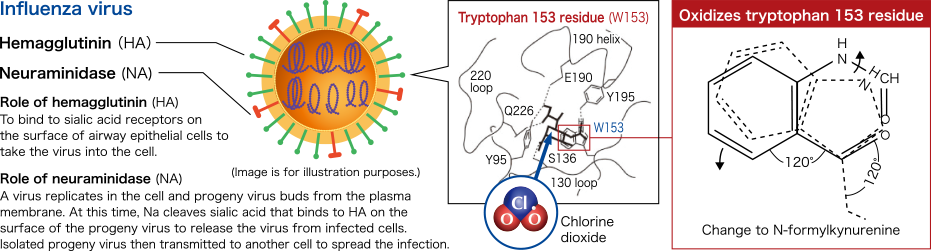
Modified from Ogata N. and Shibata T., J Gen Virol 89, 60-67 (2008). Ogata N., J Gen Virol 93, 2558-2563 (2012).
Novel coronavirus
Acts on spike proteins on viral surface and inhibits binding to ACE2 receptorsThe spike protein on the surface of the novel coronavirus (SARS-CoV-2) binds to ACE2 receptors on the surface of human epithelial cells to infect humans. Chlorine dioxide was found to act on the spike protein of the novel coronavirus (SARS-CoV-2) to inhibit the binding to ACE2 receptors.
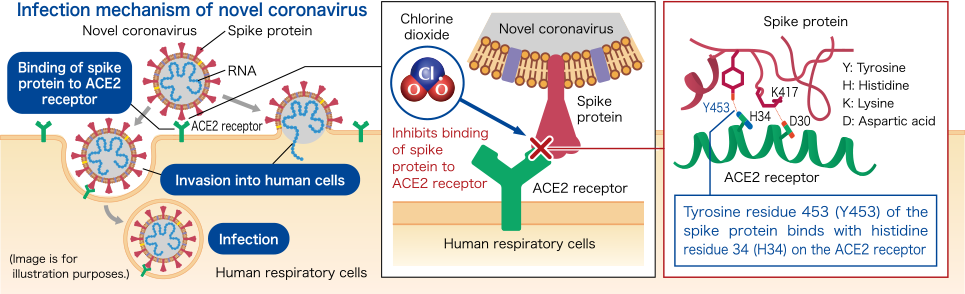
Modified from Ogata N., et al. Inhibition of the binding of spike protein of SARS-CoV-2 coronavirus to human angiotensin-converting enzyme 2 by chlorine dioxide. Ann Pharmacol Pharm 5(5), 1195. © Ogata N. 2020, Creative Commons Attribution License.
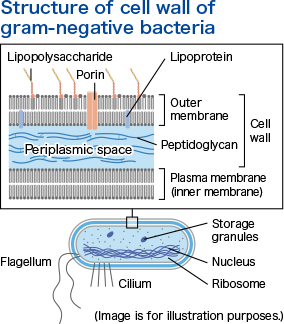
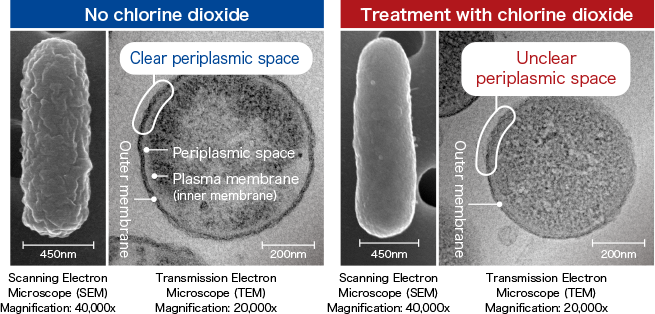
Pseudomonas aeruginosa
Changes the periplasmic space that constitutes the cell wall on the bacterial surfaceThe cell wall of gram-negative bacteria, such as Pseudomonas aeruginosa, has an outer membrane (on the outermost layer) and a layer containing peptidoglycan (between the outer membrane and the plasma membrane, also called the inner membrane). It was confirmed that the periplasmic space on Pseudomonas aeruginosa became unclear when it was treated with chlorine dioxide (100 ppm).
The 45th Annual Meeting of the Society for Antibacterial and Antifungal Agents, Japan (Tokyo, 2018)
Efficacy of chlorine dioxide gas
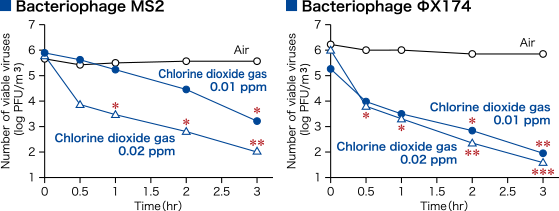
- Low-concentration chlorine dioxide gas decreased airborne viruses and bacteria.
- Ogata N., et al. Pharmacology 97, 301-306 (2016).
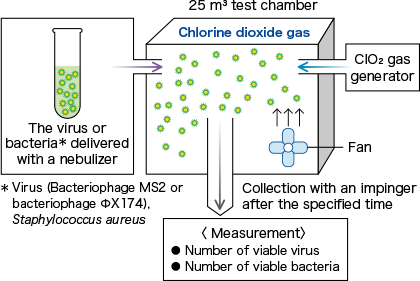
To evaluate the reducing effect for various airborne microorganisms in conditions where a low-concentration of chlorine dioxide (ClO2) gas is made to continuously present in the air in the sealed space of a 25 m3 test chamber.
ClO2 gas was released from an externally installed ClO2 gas generator (Taiko Pharmaceutical Co., Ltd.) into a closed system test chamber (25 m3), and the concentration of ClO2 gas in the chamber was adjusted to the specified concentration for the test period.
A pre-prepared virus (bacteriophage MS2 or bacteriophage ΦX174) suspension or Staphylococcus aureus suspension was delivered using a nebulizer (0.2 ml/min, 1 min) and stirred for 1 minute so that it floated in the air. After a predetermined period of time had passed, the airborne virus and bacteria were collected by an impinger and the viable virus and bacterial counts were measured.
In the control experiment, the same operations were performed under air.
chlorine dioxide gas (0.01 ppm, 0.02 ppm)
- > 2 log reduction
- > 3 log reduction
- > 4 log reduction
chlorine dioxide gas (0.01 ppm - 0.1 ppm)
- > 2 log reduction
- > 3 log reduction
- > 4 log reduction
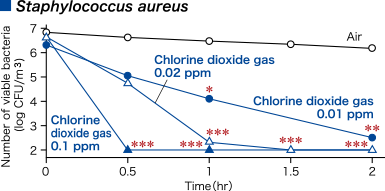
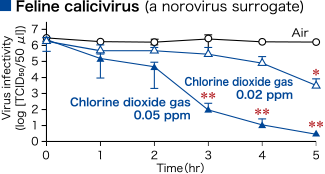
- Low-concentration chlorine dioxide gas decreased surface virus and surface bacteria.
- Morino H., Koizumi T., Miura T., Fukuda T. and Shibata T., YAKUGAKU ZASSHI 133(9), 1017-1022 (2013).
Morino H., Futatsukame M., Miura T. and Shibata T., BMC Res Note 13, 69 (2020).
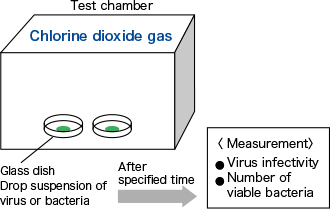
To verify the efficacy of low-concentration chlorine dioxide (ClO2) gas against various microorganisms (assuming a wet environment) dropped onto a glass dish.
A ClO2 gas generator was used to release ClO2 gas in a closed system test chamber, and the concentration of ClO2 gas in the chamber was adjusted to the specified concentration for the test period.
Glass dishes were placed inside the chamber, and 100 μL of the suspensions of various microorganisms were dropped onto them. After the specified time, the virus infectivity or the number of viable bacteria in the glass dishes was measured.
In the control experiment, the same operations were performed under air.
chlorine dioxide gas (0.01 ppm - 0.05 ppm)
- > 2 log reduction
- > 4 log reduction
Taiko Pharmaceutical Co., Ltd. investigation
Modified from Morino H., et al. YAKUGAKU ZASSHI 133,1017-1022 (2013).
chlorine dioxide gas (0.01 ppm, 0.03 ppm)
- > 2 log reduction
- > 4 log reduction
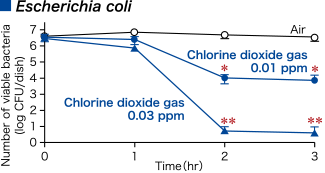
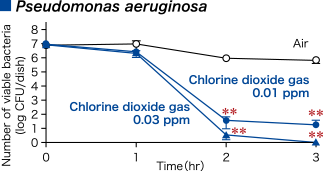
Modified from Morino H., et al. Effect of extremely low-concentration gaseous chlorine dioxide against surface Escherichia coli,Pseudomonas aeruginosa and Acinetobacter baumannii in wet conditions on glass dishes. BMC Res Notes 13, 69.© Morino H. 2020, Creative Commons Attribution License.
- Low-concentration chlorine dioxide gas inhibited the growth of filamentous fungi (Cladosporium sp.).
- Morino H., et al., The 43rd Annual Meeting of the Society for Antibacterial and Antifungal Agents, Japan (Tokyo, 2016)
To verify the efficacy of low-concentration chlorine dioxide (ClO2) gas against Cladosporium sp., which grow in wet areas in housing and are considered to be one of the causative organisms of fungal allergy.
PVC plates (fungal detectors) with mold spores and a nutritional source dripped on them were placed inside a 20 L exposure chamber, and a gas controlled to be high humidity with a ClO2 gas concentration of 0.03 ppm was introduced. Similarly, a control experiment was performed in which only air for humidity control was introduced. After 72 hours, the fungal detectors were taken out, and an evaluation was performed based on photographs and measurement of the hyphal length.
| Air | Low-concentration chlorine dioxide gas (0.03 ppm) |
|
|---|---|---|
| 0 hour |  |
 |
| After 72 hours |
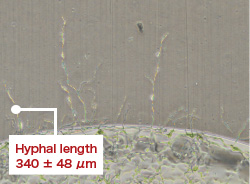 |
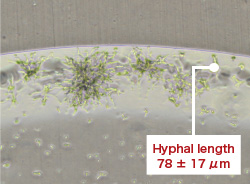 |
- Low-concentration chlorine dioxide gas prevented influenza infection in mice.
- Ogata N. and Shibata T., J Gen Virol 89, 60-67 (2008).
To verify the effect of low-concentration chlorine dioxide (ClO2) gas on the infection of mice induced by aerosols of influenza A virus.
Ten mice (CD-1 mice) were placed in each cage and either 0.03 ppm of ClO2 gas was delivered using a ClO2 gas generator, or the cage contained air alone. The influenza virus (A/PR/8/34 (H1N1), 1 LD50) was delivered in aerosol form for 15 minutes. Each mouse was then placed in an individual cage and the survival of the two groups (with delivering of 0.03 ppm ClO2 gas and with air only) was checked.
For five mice in an infection experiment under the same conditions, the virus titer (TCID50) in the lungs was measured on day 3.
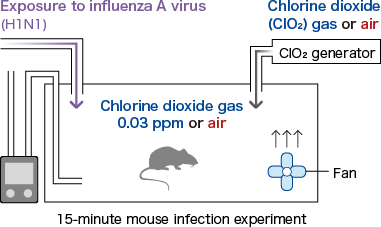
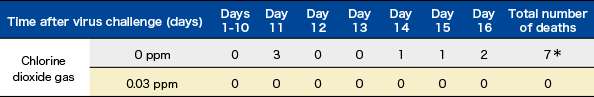
p=0.002 Comparison between 0 ppm and 0.03 ppm chlorine dioxide gas for 16 days
(Fisher's exact test, n=10 for each group)
p=0.002 Comparison between 0 ppm and 0.03 ppm chlorine dioxide gas for 16 days (Fisher's exact test, n=10 for each group)

Modified from Ogata N. and Shibata T., J Gen Virol 89, 60-67 (2008).
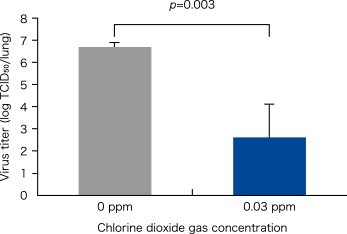
Modified from Ogata N. and Shibata T., J Gen Virol 89, 60-67 (2008).
- Interventional use of chlorine dioxide gas-generating agents significantly reduced the number of patients with influenza-like illness.
- Mimura T., Fujioka T., and Mitsumaru A., Japanese J Environ Infect 25(5), 277-280 (2010).
To investigate the preventive effect of chlorine dioxide gas-generating agent against influenza-like illness in healthy subjects.
In two adjacent buildings, a chlorine dioxide gas-generating agent (Taiko Pharmaceutical Co., Ltd.) was placed in all the rooms in one building (intervention group) and not in the other building (non-intervention group). A survey using the prospective cohort study method was then conducted of the people working in these buildings regarding the presence or absence of influenza-like symptoms* and the results of rapid influenza tests (installation period of 54 days between January 19, 2009 and March 13, 2009).
Influenza-like symptoms were defined as those meeting all of the following three conditions:
(1) Fever ≥ 38.0℃ (2) Presence of cough and/or pharyngitis (3) No cause other than influenza confirmed by medical examination and
laboratory testing methods.
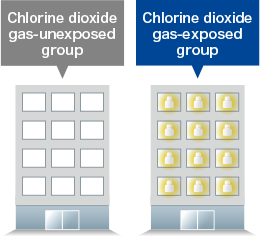
| Group with no chlorine dioxide gas-generating agent intervention | Group with chlorine dioxide gas-generating agent intervention | |
|---|---|---|
| Number of subjects | 442 subjects | 345 subjects |
| Sex ratio (male:female) | 82:18 | 93:7 |
| Mean age | 34.8 years | 43.2 years¶ |
| Vaccination rate (number of vaccinated individuals) |
23.1% (102 subjects) |
17.7% (61 subjects) |
| The presence of influenza-like symptoms |
7.2% (32 subjects) [Rapid kit positive: 2.7% (12 subjects)] |
2.3% (8 subjects) [Rapid kit positive: 0.6% (2 subjects)]¶ |
| No influenza-like symptoms | 92.8% (410 subjects) |
97.7% (337 subjects) |
Relative risk for influenza-like symptoms with chlorine dioxide gas-generating agent 0.32,
¶ p < 0.05, significantly different from the chlorine dioxide gas-unexposed group
Modified from Mimura T., et al. Japanese J Environ Infect 25(5), 277-280 (2010).
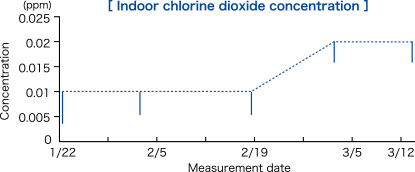
The chlorine dioxide gas-generating agents were placed in a manner that meant the rate was one chlorine dioxide gas-generating agent (stationary type 60 g) per approximately 10 m2 and one chlorine dioxide gas-generating agent (stationary type 150 g) per approximately 20 m2.
The chlorine dioxide gas-generating agent was replaced after one month. The concentration was measured in six randomly selected rooms.
The measurements were taken near the center of a room where chlorine dioxide gas-generating agent was placed (at height of about 150 cm). The mean chlorine dioxide concentration in the six rooms monitored is indicated by the dotted line, and the 95% confidence interval of the measured values is shown by the solid lines.
Modified from Mimura T., et al. Japanese J Environ Infect 25(5), 277-280 (2010).
- Chlorine dioxide gas-generating agent placed in a classroom significantly decreased the rate of absenteeism in school children.
- Ogata N. and Shibata T., Int. J. Med. Med. Sci. 1(7), 288-289 (2009).
To evaluate the effect of placing a chlorine dioxide gas-generating agent in classrooms on the attendance of school children.
Three of chlorine dioxide gas-generating agents (Taiko Pharmaceutical Co., Ltd.) were placed in a school classroom with 65-m2 floor area and school children aged 6 to 12 years for use as a deodorant. Observations were then taken of the number of absent children for 38 consecutive school days from January to March.
To examine cumulative rate of absenteeism during 38 consecutive school days for school children in classrooms with and without chlorine dioxide gas-generating agents, statistical evaluation was performed using the χ2 test.
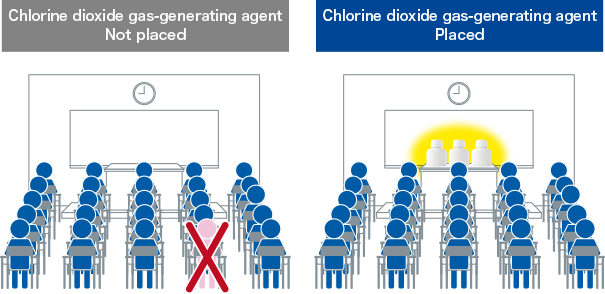
| Cumulative no. of school children | Chlorine dioxide gas-generating agent Not placed |
Chlorine dioxide gas-generating agent Placed |
|---|---|---|
| Present | 21,634 (96.0%) |
1,272 (98.5%) |
| Absent | 900 (4.0%*) |
20 (1.5%*) |
* p < 0.00001、χ2 test
Modified from Ogata N., et al. Effect of chlorine dioxide gas of extremely low concentration on absenteeism of schoolchildren. Int. J. Med. Med. Sci. 1(7), 288-289,© Ogata N., et al. 2009, Creative Commons Attribution License.
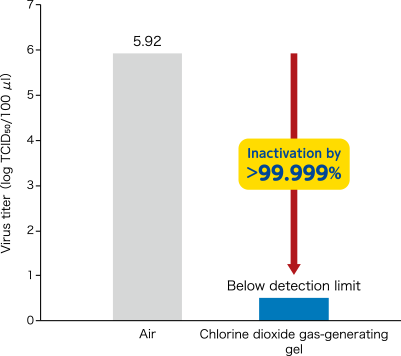
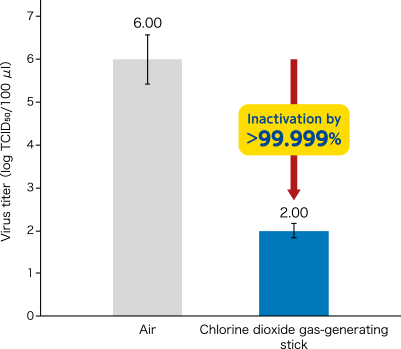
- Inactivation effect of novel coronavirus (SARS-CoV-2) by chlorine dioxide gas was confirmed.
- Taiko Pharmaceutical Co., Ltd. investigation
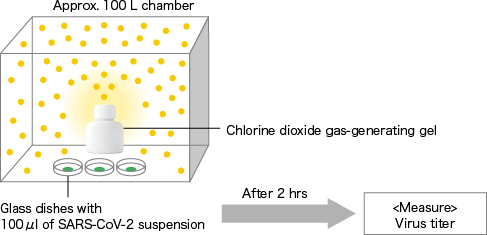
Chlorine dioxide gas-generating gel
[ Method ]A chlorine dioxide gas-generating gel (150 g) was put into approximately 100 L plexiglass sealed chamber. After ClO2 gas was generated for 24 hours, three glass dishes with 100 μl of novel corona virus (SARS-CoV-2/human/CHN/SH01/2020) suspension dripped were placed in the chamber. After 2 hours, the surviving virus (TCID50) in the dishes was quantitated. Virus titer was determined by 50% tissue culture infective dose per 100 μl (TCID50/100 μl). In a control experiment, the same operation was performed under air. Data represented mean ± standard deviation (n=3).
[The chlorine dioxide gas-generating gel manufactured by Taiko Pharmaceutical Co., Ltd. was used.]
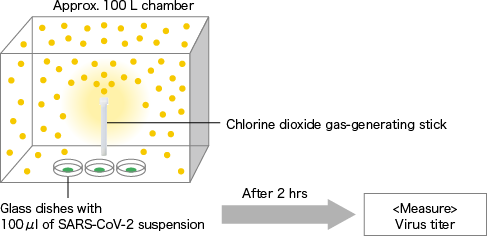
Chlorine dioxide gas-generating stick
[ Method ]A chlorine dioxide gas-generating stick was put into approximately 100 L plexiglass sealed chamber. After ClO2 gas was generated, three glass dishes with 100 μl of novel corona virus (SARS-CoV-2/human/CHN/SH01/2020) suspension dripped were placed in the chamber. After 2 hours, the surviving virus (TCID50) in the dishes was quantitated. Virus titer was determined by 50% tissue culture infective dose per 100 μl (TCID50/100 μl). In a control experiment, the same operation was performed under air. Data represented mean ± standard deviation (n=3).
[The chlorine dioxide gas-generating stick (G stick) manufactured by Taiko Pharmaceutical Co., Ltd. was used.]
Safety of chlorine dioxide gas
In terms of occupational safety and health, the Occupational Safety and Health Administration of the United States Department of Labor (U.S. OSHA) has established the permissive exposure level for inhaled chlorine dioxide 1).
| PEL-TWA | Time-weighted average level with no adverse effect on workers in repeated work for eight hours per day, 40 hours per week | |
|---|---|---|
| PEL-STEL | Typical 15-minute time-weighted average permissible limit |
In Japan, the guideline value for indoor level of chlorine dioxide gas has been set by the Japan Chlorine Dioxide Industry Association from the viewpoint of the safe use of chlorine dioxide gas products 2).
| Guideline value for indoor level of chlorine dioxide gas | The value for the concentration in air at which it is considered unlikely that the health of a human would be adversely affected, even if the person continues to inhale that concentration for a lifetime |
|---|
Taiko Pharmaceutical Co., Ltd. conducted the following safety evaluations in animal studies.
※The table can be scrolled horizontally.
| Chronic toxicity 3) | Chronic toxicity 3) | Repeated dose toxicity 4) | |
|---|---|---|---|
| Testing institute | HAMRI Co., Ltd. | HAMRI Co., Ltd. | HAMRI Co., Ltd. |
| Test summary | Rats were systemically exposed to chlorine dioxide gas at a mean concentration of 0.05 ppm continuously for six months. | Rats were systemically exposed to chlorine dioxide gas at a mean concentration of 0.1 ppm continuously for six months. | Rats were systemically exposed to chlorine dioxide gas at a mean concentration of 1 ppm for 5 hours per day, 5 days per week for 10 weeks. |
| Results | No abnormalities were noted in body weight, biochemistry, hematology, autopsy, organ weight, or histopathology. | No abnormalities were noted in body weight, biochemistry, hematology, autopsy, organ weight, or histopathology. | No abnormalities were noted in body weight, biochemistry, hematology, autopsy, organ weight, or histopathology. |
- 1) Occupational Health Guideline for Chlorine Dioxide: the Occupational Safety and Health Administration of the United States Department of Labor (1978).
- 2) Japan Chlorine Dioxide Industry Association: Evaluation of chlorine dioxide for the setting of voluntary management criteria (2014).
- 3) Akamatsu A., et al. J Occup Med Toxicol 7:2, (2012).
- 4) Ogata N., et al. J Drug Metab Toxicol 4(2), (2013).
Safety and efficacy of chlorine dioxide gas
※The table can be scrolled horizontally.
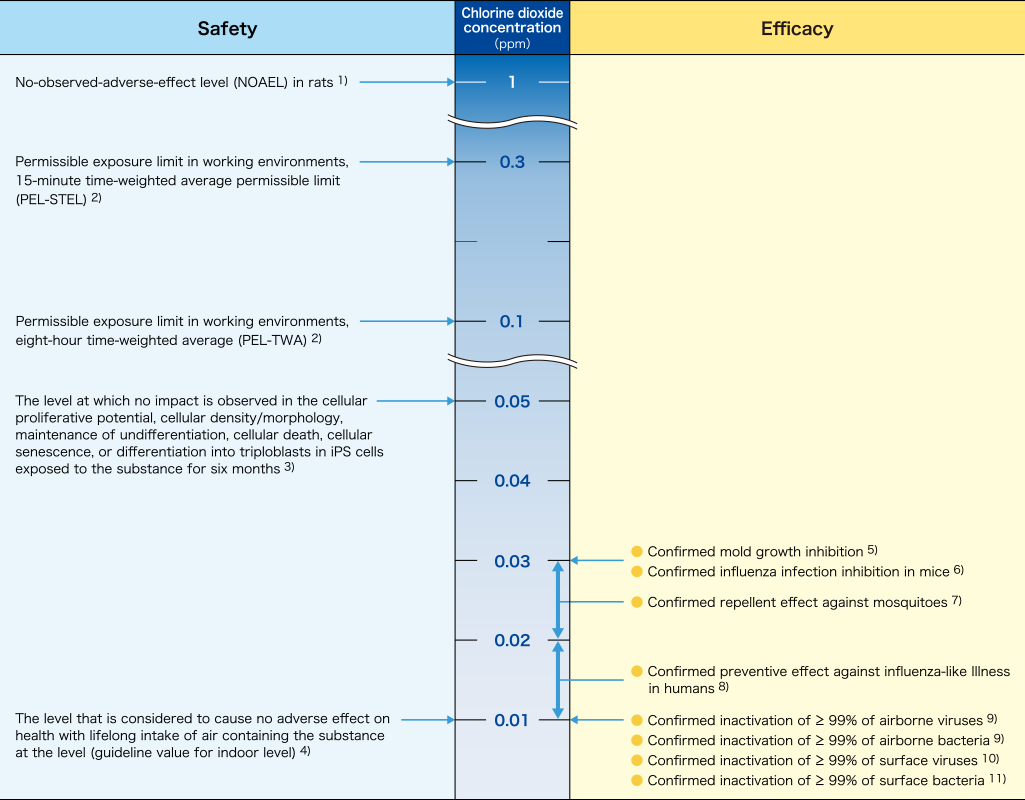
- 1) Ogata N., et al. J Drug Metab Toxicol 4(2), (2013).
- 2) Occupational Health Guideline for Chlorine Dioxide: the Occupational Safety and Health Administration of the United States Department of Labor (1978).
- 3) Graduate School of Medicine, Osaka University joint research course on spatial environment infection control, 19th Congress of the Japanese Society for Regenerative Medicine (2020).
- 4) Japan Chlorine Dioxide Industry Association: Evaluation of chlorine dioxide for the setting of voluntary management criteria (2014).
- 5) Morino H., et al. The 43rd Annual Meeting of the Society for Antibacterial and Antifungal Agents, Japan (2016).
- 6) Ogata N. and Shibata T. J Gen Virol 89, 60-67(2008).
- 7) Matsuoka H. and Ogata N. Med Entomol Zool 64(4), 203-207(2013).
- 8) Mimura S., et al. Japanese J Environ Infect 25 (5), 277 -280 (2010).
- 9) Ogata N., et al. Pharmacology 97, 301-306(2016).
- 10) Taiko Pharmaceutical Co., Ltd. investigation
- 11) Morino H., et al. BMC Res Notes 13, 69(2020).





